WASHINGTON — A rule proposed by the US Food and Drug Administration (FDA) would establish a list of foods that would require additional recordkeeping beyond what existing regulations require.
Frank Yiannas, deputy commissioner for Food Policy and Response at the FDA, said the “Requirements for Additional Traceability Records for Certain Foods” proposed rule for food traceability is a critical step forward in the agency’s efforts to bring about farm-to-table traceability in the US food supply. The rule also would help the agency rapidly identify recipients of those foods to prevent or mitigate foodborne illness outbreaks.
“The FDA has proposed a new rule that lays the foundation for the end-to-end food traceability across the food industry that we’ll be working toward over the next decade as part of the New Era of Smarter Food Safety initiative,” Mr. Yiannas said. “When we say traceability, what we are really talking about is the ability to track a food at every step of the supply chain. While limited to certain foods, this proposed rule would create a first-of-its-kind standardized approach to traceability recordkeeping, paving the way for industry to adopt and leverage more digital, tech-enabled traceability systems both in the near term and the future.”
For example, shell eggs, ready-to-eat deli salads and cheeses other than hard cheeses were identified for inclusion on the Food Traceability List (FTL). The FDA identified foods to include on the FTL based on seven criteria:
- Frequency of outbreaks and occurrences of illnesses;
- Severity of illnesses;
- Likelihood of contamination;
- The potential for pathogen growth, with consideration of shelf life;
- Manufacturing process contamination probability and industry-wide intervention;
- Consumption rate and amount consumed; and
- Cost of illness
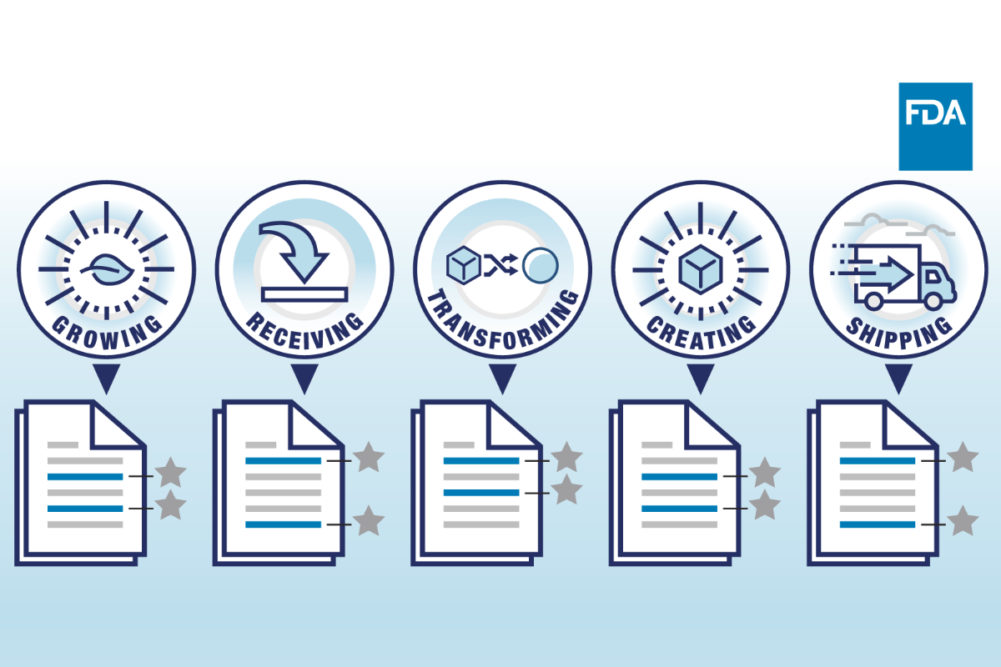
The FTL is the core component of the “Requirements for Additional Traceability Records for Certain Foods.” Companies that process, manufacture, pack or hold foods included on the FTL are to establish and maintain records associated with Critical Tracking Events (CTE) such as growing, receiving, transforming, creating, and shipping of foods on the FTL. The rule also establishes exemptions for farms that sell directly to consumers and a partial exemption for retail food establishments among several other special circumstances.
“While the proposed requirements would only apply to those foods on the FTL, they were designed to be suitable for all FDA-regulated food products,” the agency said. “FDA would encourage the voluntary adoption of these practices industry-wide.”
The proposed rule will be available for public comment for 120 days following publication in the Federal Register.