WASHINGTON — The US Food and Drug Administration on Sept. 18 announced the latest in a series of initiatives aimed at providing temporary relief from enforcement of certain regulations and deadlines to food manufacturers struggling to adjust operations and procedures to ensure food is directed safely and in the quantities and forms required during the coronavirus (COVID-19) pandemic.
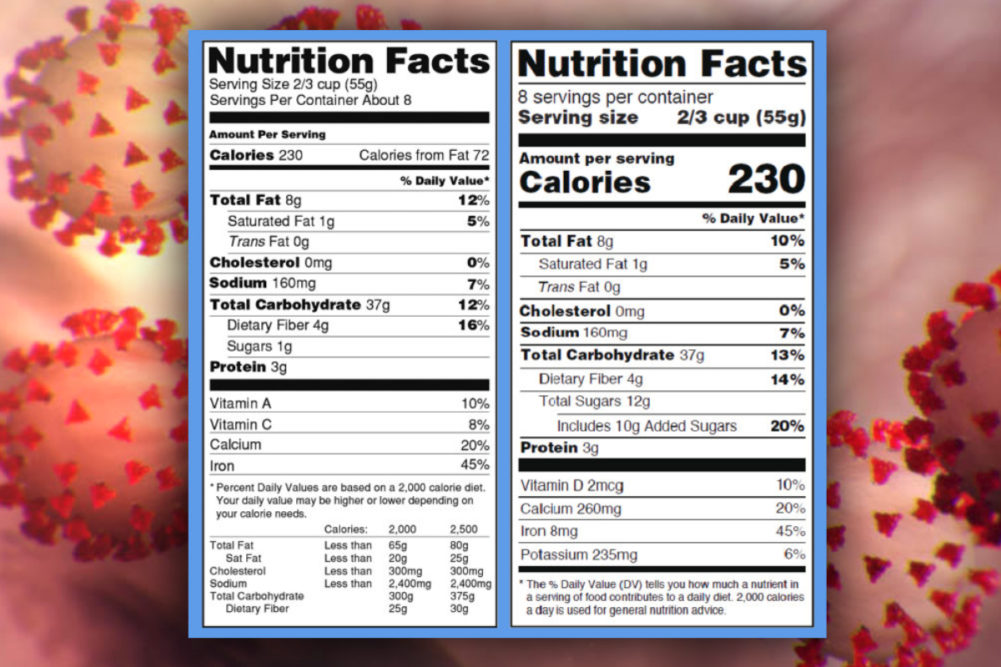
In its latest announcement, the FDA said it will provide additional flexibility for manufacturers who need to comply with updated Nutrition and Supplement Facts label requirements by Jan. 1, 2021. The upcoming compliance date applies to manufacturers with less than $10 million in annual food sales.
The FDA said although the compliance date will remain in place, the agency will not focus on enforcement actions during 2021 for these smaller food manufacturers.
The FDA noted it provided the same flexibility to manufacturers with $10 million or more in annual sales, who were required to comply with the Nutrition and Supplement Facts label requirements by Jan. 1, 2020, by indicating it would not focus on enforcement actions during 2020.
The eruption of the COVID-19 pandemic in the United States upended a finely honed food production and distribution system, throwing food manufacturers and distributors and entire sectors, such as foodservice, off balance.
The FDA, the US Department of Agriculture’s Food Safety and Inspection Service and several other federal agencies have introduced temporary measures aimed at ensuring food and other essential products reach the consumers in the most efficient manner possible while not compromising food or employee safety.
In the last few months, the FDA has announced regulatory changes that will be in effect for the duration of the pandemic. It has suspended routine food facility inspections. It has allowed food initially packaged to supply the foodservice sector to be redirected to the retail sector, which was crucial when most of the nation went into lockdown and consumers depended on food they could prepare at home. It has allowed minor formulation changes in the event food manufacturers found they were not able to procure the specific ingredients they required, without making labeling changes.
These and other temporary changes have allowed the food industry to successfully respond to the crisis.
“What was appreciated by industry was not only what FDA did, but what other regulatory agencies did as well,” Betsy Booren, PhD, senior vice president, regulatory and technical affairs, Consumer Brands Association, told Food Business News. “The flexibilities they announced allowed consumer products, whatever they might be, to get to consumers sooner.”
Dr. Booren provided a couple of examples. She pointed out the pandemic emerged just as baseball season was set to begin, and a lot of extra food was being directed to the foodservice sector. She credited the FDA and the FSIS for providing, within parameters, the flexibility the food industry required to reposition that food and direct it into retail marketing channels.
“The food industry was able to provide a lot of good food to retailers and consumers and prevent wastage,” she said.
She also discussed the FDA’s temporary flexibility that allowed food manufacturers to substitute similar minor ingredients for original ingredients that could not be accessed because of disrupted supply lines, as long as there was not an allergen or other food safety issue.
“The example given by FDA was if you used unbleached flour, but you couldn’t get it, you could use bleached flour,” Dr. Booren said. “Typically, that would require labeling changes. This flexibility allowed core products to continue to be produced and shipped.”
Dr. Booren said some of the temporary policies put into place for COVID-19 may stimulate discussion on what in them might be worth retaining or at the least what might be learned from the experience.
For instance, with regard to food production facility inspections and audits, in-person inspections are necessary and will continue, Dr. Booren said, “but can we streamline unnecessary in-person interaction” by employing new technologies.
Temporary is the key word in the FDA’s announced flexibilities, and it is important to keep that in mind, David Acheson, president and chief executive officer, The Acheson Group, told Food Business News.
“The FDA has been flexible on routine work but is chasing for-cause work and follow-ups on prior investigations and outbreaks,” Mr. Acheson said. “Domestically, they will likely ramp up routine inspections slowly and eventually have to get back to the congressionally mandated level of inspections — but that may take a while.”
Mr. Acheson noted there also has been flexibility on some aspects of the FDA’s Foreign Supplier Verification Program.
“Yet, there have been plenty of warning letters around FSVP to importers,” he said. “I think their (the FDA’s) routine foreign inspections will come back, but not until COVID-19 is much more under control.
“So, there have been some short-term changes, but I don’t see these going long term.”